Administer Flagyl IV over at least one hour. This ensures patient comfort and minimizes the risk of adverse reactions. Shorter infusion times can lead to phlebitis.
The recommended dosage varies based on the specific infection being treated and the patient’s weight and overall health. Always consult the official prescribing information for accurate dosage guidelines. Never deviate from prescribed protocols without explicit medical direction.
Closely monitor the patient for any signs of infusion-related reactions, such as fever, chills, or nausea. Immediate cessation of the infusion is necessary if any significant adverse events occur. Post-infusion observation is also recommended to detect delayed reactions.
Proper aseptic technique during preparation and administration is paramount to prevent infection. Adhere strictly to established guidelines for handling and disposing of intravenous medications. Remember to document the infusion time, dosage administered, and patient response carefully in the medical record.
Note: This information is for educational purposes only and does not constitute medical advice. Always consult a healthcare professional for guidance on the administration of Flagyl or any other medication.
- Flagyl IV Administration Time: A Comprehensive Guide
- Dilution Guidelines
- Monitoring the Patient
- Dosage Adjustments
- Additional Information
- Understanding Flagyl’s IV Infusion Rate
- Adjusting Infusion Time Based on Dosage
- Monitoring the Patient
- Calculating Infusion Time Based on Dose and Concentration
- Common IV Administration Sets and Their Impact on Infusion Time
- Monitoring the Patient During Flagyl IV Infusion
- Addressing Potential Complications and Side Effects
- Gastrointestinal Issues
- Neurological Effects
- Hematological Effects
- Allergic Reactions
- Other Potential Complications
- Patient Education
- Post-Infusion Care and Follow-up
Flagyl IV Administration Time: A Comprehensive Guide
Administer Flagyl IV over at least one hour. This ensures patient safety and minimizes potential adverse effects. For a 500mg dose, a rate of 500mg/hour is recommended. Always consult the medication’s package insert for specific dilution and administration instructions; they may vary slightly depending on the formulation.
Dilution Guidelines
Correct dilution is critical. Use compatible intravenous fluids like dextrose 5% in water (D5W) or normal saline (0.9% NaCl). Never use solutions containing electrolytes incompatible with Flagyl. Prepare the solution aseptically and inspect for particulate matter or discoloration before administering.
Monitoring the Patient
Closely monitor the patient during and after infusion for signs of infusion-related reactions such as phlebitis or allergic reactions. Observe for any signs of nausea, vomiting, diarrhea, or changes in liver function tests, particularly if administering high doses or prolonged treatment. Document all observations and promptly report any unexpected adverse events.
Dosage Adjustments
Dosage adjustments are necessary for patients with impaired renal or hepatic function. Consult prescribing information for specific dosage modifications based on creatinine clearance or liver function test results. Always prioritize patient safety and adjust the infusion rate as needed.
Additional Information
Before initiating Flagyl IV therapy, obtain a complete patient history, including allergies and concurrent medications. This allows for appropriate precaution and informed decision-making. Always verify the patient’s identity using two patient identifiers before administering any medication.
Understanding Flagyl’s IV Infusion Rate
Metronidazole (Flagyl) IV infusion should generally occur over at least 30 minutes. This minimizes the risk of adverse effects.
Adjusting Infusion Time Based on Dosage
For higher doses, extending the infusion time beyond 30 minutes, potentially to an hour, is recommended. Always follow your physician’s specific instructions and the prescribing information provided with the medication. Never exceed the maximum recommended daily dose.
Monitoring the Patient
Closely monitor patients during and after Flagyl infusion for any signs of adverse reactions, including nausea, vomiting, or allergic responses. Immediate intervention is crucial if such reactions occur. Be prepared to adjust the infusion rate or stop the infusion based on the patient’s response.
Calculating Infusion Time Based on Dose and Concentration
First, determine the total dose of Metronidazole needed. Let’s say your order is for 500mg.
Next, identify the concentration of your Metronidazole IV solution. Common concentrations are 500mg/100ml or 5mg/ml. Suppose you have a 500mg/100ml bag.
Now, calculate the infusion volume. In this example, you need the entire 100ml bag to administer the 500mg dose.
Finally, determine the infusion rate. A typical infusion time for 500mg Metronidazole in 100ml is 30-60 minutes. Always refer to your institution’s guidelines and the drug’s prescribing information for specific infusion rate recommendations. A faster infusion rate may be appropriate under some circumstances; a slower rate might be chosen for patient comfort. Remember to always double-check your calculations with a colleague before administering the medication.
If you have a different concentration or dose, use the following formula: Infusion time (minutes) = (Volume in ml) / (Infusion rate in ml/min). For example, if your solution is 250mg/50ml and you need 250mg, the infusion volume is 50ml. With an infusion rate of 50ml/30 minutes, the infusion time is 30 minutes.
Always carefully review the medication order and patient’s chart before beginning the infusion. Accurate calculations and cautious administration are paramount.
Common IV Administration Sets and Their Impact on Infusion Time
Choosing the right IV administration set significantly affects Metronidazole infusion time. Faster infusion rates generally require sets with smaller drip chambers and tubing with a smaller internal diameter.
| Administration Set Type | Drip Chamber Size (mL) | Tubing Internal Diameter | Approximate Infusion Time (for 500mg Metronidazole in 100mL D5W) |
|---|---|---|---|
| Standard Micro-drip Set | 15-20 | Small | 60-90 minutes |
| Macro-drip Set | 50-100 | Larger | 30-60 minutes (requires faster infusion pump settings and careful monitoring) |
| Volume-Control Infusion Set | 50-150 | Variable | Adjustable, typically 60-120 minutes, depending on set volume and infusion rate. |
Note: These times are estimates. Actual infusion time depends on factors such as pump settings, patient factors, and the specific Metronidazole concentration. Always refer to the manufacturer’s instructions for your specific IV set and adhere to institutional guidelines.
For example, a microdrip set provides greater control over slower infusions and is often preferred for Metronidazole, allowing for more precise rate management. However, a macro-drip set might be suitable with a controlled infusion pump for quicker administrations when appropriate. Remember that using an infusion pump is generally recommended for administering Metronidazole intravenously to ensure accurate and safe delivery.
Consult your hospital’s formulary and guidelines for specific recommendations regarding appropriate IV sets for Metronidazole administration.
Monitoring the Patient During Flagyl IV Infusion
Closely observe the patient for signs of infusion-related reactions. Monitor vital signs, including blood pressure, heart rate, and respiratory rate, every 15 minutes during the first hour and then hourly thereafter. Pay particular attention to any changes from baseline values.
Assess for signs and symptoms of allergic reactions, such as rash, itching, swelling, or difficulty breathing. Report any such occurrences immediately to the physician.
- Medication administration: Administer Flagyl IV slowly over at least 30 to 60 minutes to minimize the risk of adverse effects. Rapid infusions can lead to phlebitis.
- Fluid balance: Monitor hydration status. Adequate hydration is important for optimal drug distribution and to reduce the risk of nephrotoxicity.
- Neurological status: Assess for neurological changes, including dizziness, confusion, or seizures. These are rare but possible adverse effects.
Document all observations, including vital signs, infusion rate, and any adverse events. Report any significant changes to the physician or healthcare provider.
- Observe for signs of thrombophlebitis at the infusion site: redness, swelling, pain, or warmth. If noted, discontinue the infusion and notify the physician immediately.
- Monitor laboratory values, specifically complete blood counts (CBC), to detect any hematological changes.
- Regularly assess the infusion site for any signs of infiltration or extravasation. Take appropriate action if such complications occur.
Patient education is key. Instruct patients about potential side effects and the importance of reporting any unusual symptoms.
Addressing Potential Complications and Side Effects
Monitor patients closely for signs of infusion-related reactions, such as phlebitis. Slow the infusion rate if these occur. Discontinue infusion and notify the physician immediately if serious reactions like anaphylaxis develop.
Gastrointestinal Issues
- Nausea and vomiting are common. Consider administering antiemetics prophylactically, particularly in patients with a history of gastrointestinal sensitivity.
- Diarrhea can occur. Adequate hydration is crucial. Severe or persistent diarrhea warrants medical attention.
- Pseudomembranous colitis, a serious complication, requires immediate medical intervention. This is characterized by severe diarrhea, abdominal cramping, and fever. Treatment may include discontinuation of Flagyl and administration of antibiotics.
Neurological Effects
Peripheral neuropathy, characterized by numbness, tingling, or pain in the extremities, is a possible side effect. Regular neurological assessments are recommended, especially during prolonged treatment. Consider adjusting dosage or discontinuing the drug if neuropathy develops.
Hematological Effects
- Monitor complete blood counts (CBC) regularly, especially in patients with pre-existing hematological conditions. Flagyl can affect white blood cell counts.
- Reduce dosage or discontinue treatment if significant changes in blood counts are observed. Closely collaborate with the hematology team in such cases.
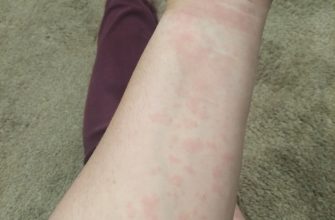
Allergic Reactions
Although rare, allergic reactions can range from mild skin rashes to severe anaphylaxis. Have appropriate emergency medications readily available. Patients should be instructed to report any new or worsening skin reactions.
Other Potential Complications
- Monitor liver function tests (LFTs) periodically, especially in patients with liver impairment. Hepatotoxicity is a rare but potential side effect.
- Assess for signs of seizures, particularly in patients with a seizure history or those receiving concurrent medications that lower the seizure threshold.
Patient Education
Educate patients about potential side effects and encourage them to report any unusual symptoms. Emphasize the importance of completing the prescribed course of treatment, even if they feel better.
Post-Infusion Care and Follow-up
Monitor the patient for at least 30 minutes post-infusion for any immediate adverse reactions, such as allergic responses or infusion site complications. Observe for symptoms like rash, itching, swelling, or difficulty breathing.
Ensure adequate hydration by encouraging fluid intake. This helps with medication clearance.
Educate the patient about potential side effects, including nausea, vomiting, diarrhea, and metallic taste. Explain how to manage mild side effects and when to seek immediate medical attention (e.g., severe diarrhea, persistent vomiting).
Instruct the patient to report any new or worsening symptoms to their healthcare provider. This includes unusual bruising, bleeding, or changes in bowel movements.
Schedule a follow-up appointment to assess the effectiveness of treatment and monitor for any complications. The frequency of follow-up appointments will depend on the patient’s condition and response to treatment.
Provide clear written instructions summarizing post-infusion care and follow-up instructions. This should include contact information for their healthcare provider or emergency services.
Note: Always adhere to the specific instructions provided by the prescribing physician.
Remember: Patient safety and well-being are paramount. Thorough monitoring and education are key to a successful treatment course.